PV diagram
The PV diagram models the relationship between pressure (P) and volume (V) for an ideal gas. An ideal gas is one that never condenses regardless of the various changes its state variables (pressure, volume, temperature) undergo. In addition, the processes plotted on PV diagrams only work for a closed system (in this case the ideal gas), so there is no exchange of matter, but still an exchange of energy.
Pressure and volume exhibit a causal relationship, meaning that the change of one variable will cause a change in the other. To understand how pressure directly affects volume (and vice versa)—imagine a sealed container, containing an ideal gas (the system), that has a moving piston. If a force is applied, the piston moves down, and the gas would compress—decreasing the volume in the system and causing an increase in pressure. Moreover, if the piston moves up, the volume of the system would increase, decreasing the pressure of the system. Therefore, an increase in one variable will cause a decrease in the other, and vice versa. However, if an increase (or decrease) in pressure and/or volume is desired, an external heat source (or a cooling source) from its surroundings must be added.
In addition, these diagrams not only model the relationship between pressure and volume for an ideal gas but can also be used to calculate work done (on or by the system). This is done by calculating the negative of the area under the curve which can be done geometrically or by integration. The general formula to calculate work done by an ideal gas is integral.
where:
: means that work is being done on the system (compression)
: means that work is being done by the system (expansion)
: no work is done (isochoric process—see figure below)
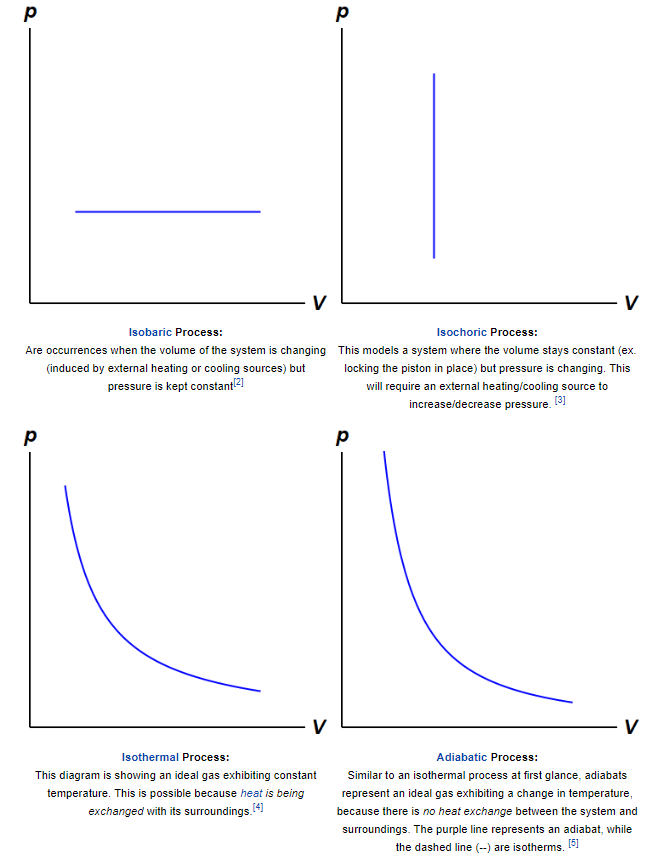
The fundamental thermodynamic processes modeled on PV diagrams (isochoric, isobaric, and isothermal processes) all follow the ideal gas law except for adiabatic processes—which will be discussed in detail on its main page. The following are examples of each process modeled on the PV diagram. Each of their pages will describe their unique characteristics in detail:
Four main ideal gas law processes modeled on PV diagrams
Creating PV diagrams for isothermal processes
Using the rules above, we can create diagrams for an isothermal process of expansion and compression.
- Diagram 3 (the top diagram in the set of diagrams below) shows isothermal expansion. In this case, the expansion comes with a decrease in pressure from p1 to p2 and a volume increase from V1 to V2.
- Diagram 3 (the bottom diagram in the set of diagrams below) shows isothermal compression, and the inverse process occurs: the volume decreases from V1 to V2 and the pressure increases from p1 to p2.
 Diagram 3. Isothermal expansion is shown in the first part of the diagram, and isothermal compression is shown in the second part. Manuel R. Camacho – StudySmarter Originals
Diagram 3. Isothermal expansion is shown in the first part of the diagram, and isothermal compression is shown in the second part. Manuel R. Camacho – StudySmarter Originals
For isothermals (isothermic process lines), larger temperatures will be further away from the origin. As the diagram below shows, temperature T2 is larger than temperature T1, which is represented by how far they are from their origin.

For More Press Here